Risk adjusted net present value: What is the current valuation of Eli Lilly and Co’s Retatrutide?
Pharmaceutical Technology
FEBRUARY 24, 2023
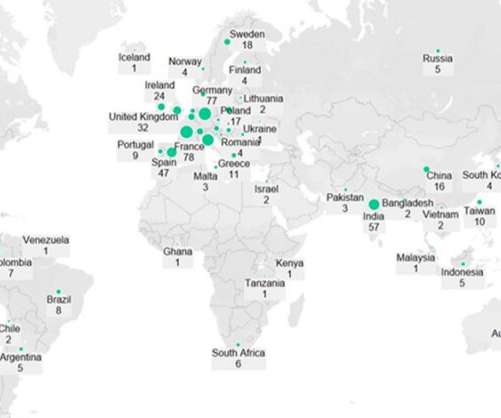
According to Globaldata, it is involved in 10 clinical trials, of which 4 were completed, 5 are ongoing, and 1 is planned. GlobalData uses proprietary data and analytics to provide a complete picture of Retatrutide’s valuation in its risk-adjusted NPV model (rNPV). It is a new molecular entity. million in FY2021.

























Let's personalize your content